Inhibiting an astrocytic ‘brake’ that blocks spinal cord repair could pave path to neuronal regeneration

Spinal cord injuries caused by external trauma, such as traffic accidents or falls, often lead to the permanent loss of motor and sensory functions. This is because the spinal cord—the central pathway connecting the brain and the rest of the body—harbors a “brake” mechanism that halts repair. For the first time, the molecular mechanism behind this braking system has been revealed.
A research team led by Director C. Justin Lee of the Center for Cognition and Sociality at the Institute for Basic Science (IBS), in collaboration with Professor Ha Yoon of Yonsei University College of Medicine, identified that the inhibitory neurotransmitter GABA, produced by astrocytes in the spinal cord through the enzyme monoamine oxidase B (MAOB), is the key factor that blocks recovery after spinal cord injury. Furthermore, the team demonstrated the therapeutic potential of targeting this pathway by showing that MAOB inhibition promotes spinal cord repair.
The work appears in Signal Transduction and Targeted Therapy.
Until now, recovery failure after spinal cord injury has largely been attributed to the formation of the so-called glial barrier. This barrier, formed by the rapid proliferation of astrocytes and other glial cells around the lesion, protects the injury site in the acute phase, but later prevents axonal regrowth. However, the precise molecular mechanism hindering regeneration had remained unclear. As a result, existing treatments for spinal cord injury have primarily focused on suppressing inflammation or alleviating symptoms, rather than directly addressing neural repair.
Building on their earlier work, which showed that reactive astrocytes abnormally produce GABA via MAOB and exacerbate neurodegenerative diseases such as Alzheimer’s, the team investigated whether a similar mechanism occurs in spinal cord injury. They found that GABA suppresses the expression of BDNF (a key neurotrophic factor) and its receptor TrkB, both essential for neuronal regeneration. Consequently, the production of GABA acts as a molecular brake, shutting down growth signals and blocking axonal regrowth and functional recovery after injury.

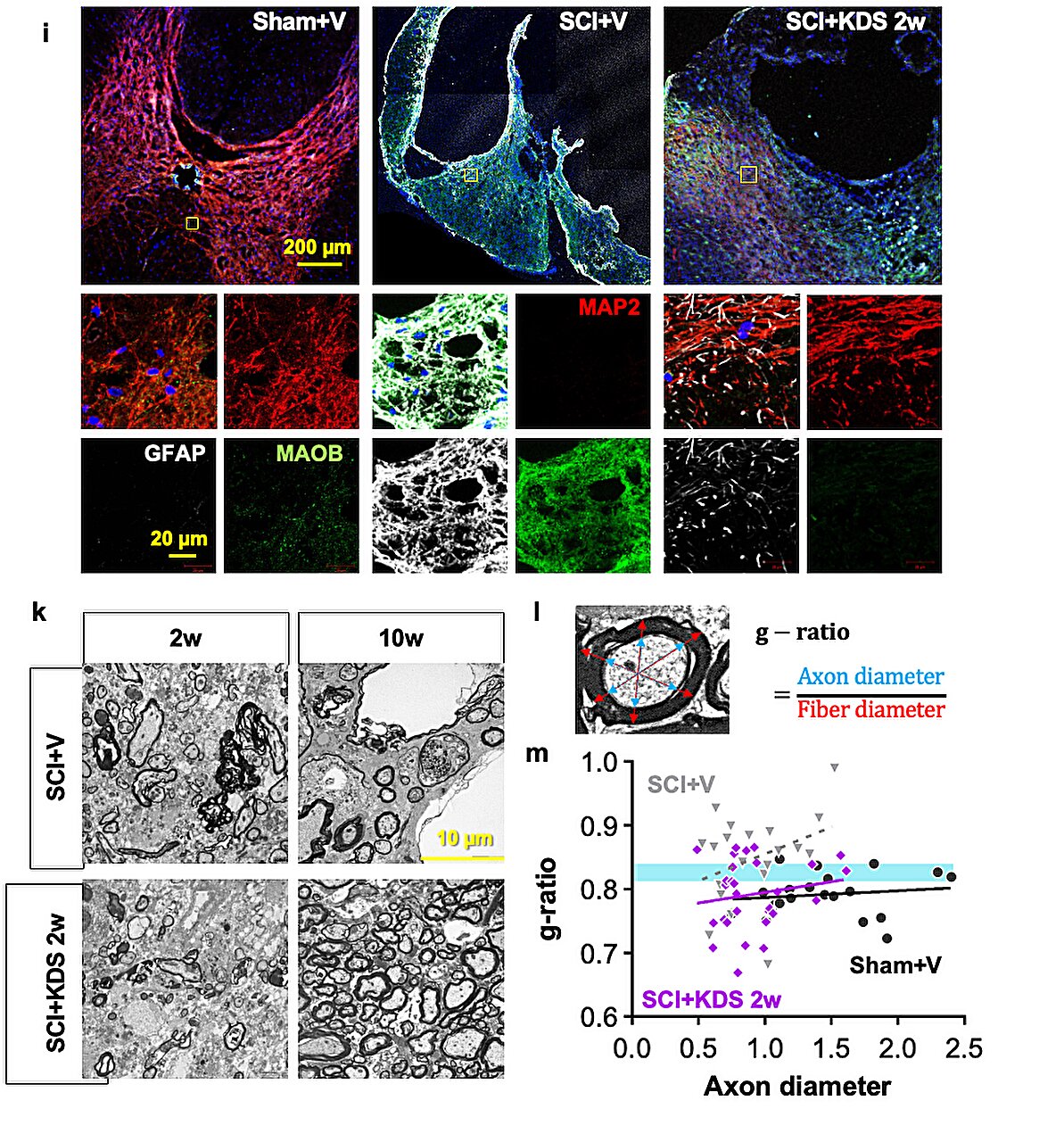
To validate this, the researchers used animal models in which MAOB expression in spinal astrocytes was either suppressed or enhanced. Inhibition of MAOB allowed axons to regrow and restored hindlimb motor function, while increased MAOB expression led to severe tissue loss and almost no functional recovery. These findings confirmed that the MAOB–GABA pathway directly prevents spinal cord regeneration.
The team further tested the MAOB inhibitor KDS2010 in animal models of spinal cord injury. Treated mice showed significant improvements in locomotion, such as fewer hindlimb slips in ladder-walking tests, and exhibited robust axonal regrowth at the injury site. Histological analyses revealed reduced lesion cavities and increased remyelinated axons.
Importantly, similar benefits were confirmed in non-human primates, where tissue preservation and neural protection were markedly improved. The safety and tolerability of KDS2010 had already been validated in a Phase I clinical trial in healthy adults, underscoring its potential as a therapeutic candidate.
Director C. Justin Lee of IBS stated, “This study identifies a direct molecular pathway that suppresses neural regeneration after spinal cord injury and presents a strategy to overcome it. Unlike existing treatments, this offers a fundamentally new therapeutic approach. The multi-level validation in rodents, primates, and Phase I clinical trials provides strong evidence that this drug candidate could translate into real treatment for patients.”
Professor Ha Yoon of Yonsei University College of Medicine added, “KDS2010 has already demonstrated safety in a Phase I clinical trial, and we plan to proceed with Phase II trials to evaluate its efficacy in spinal cord injury patients. Moreover, we aim to investigate whether the MAOB–GABA pathway plays a role in other neurological disorders, broadening its potential applications into a more comprehensive therapeutic platform.”
More information:
Astrocytic monoamine oxidase B (MAOB)–gamma-aminobutyric acid (GABA) axis as a molecular brake on repair following spinal cord injury, Signal Transduction and Targeted Therapy (2025). DOI: 10.1038/s41392-025-02398-2
Citation:
Inhibiting an astrocytic ‘brake’ that blocks spinal cord repair could pave path to neuronal regeneration (2025, September 10)
retrieved 10 September 2025
from https://medicalxpress.com/news/2025-09-inhibiting-astrocytic-blocks-spinal-cord.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.